
Renal Cell Cancer Subcommittee
ANNUAL
REPORT 2024


Renal Cell Cancer Subcommittee
ANNUAL
REPORT 2024

ANNUAL REPORT 2024
Renal Cell Cancer Subcommittee
Renal Cell Cancer Subcommittee

Craig Gedye
CHAIR

David Pook
DEPUTY CHAIR
The Renal Cell Cancer Subcommittee has demonstrated exceptional productivity last year. Below, we outline a summary of our accomplishments.
Trial Updates
KEYPAD (ANZUP 1601)
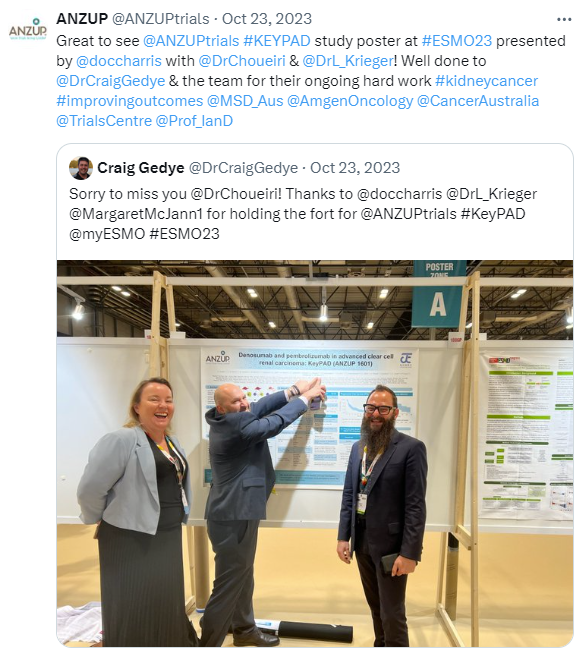
This trial is now closed to recruitment. Data cleaning and analysis are currently underway, and we submitted an abstract for the European Society for Medical Oncology (ESMO) 2023 conference which was presented as a poster: ‘Denosumab and pembrolizumab in advanced clear cell renal carcinoma: KeyPAD (ANZUP 1601)’.
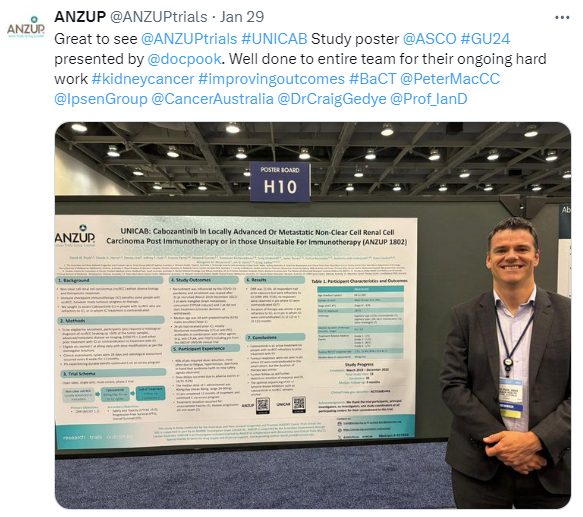
UNICAB (anzUp Non-clear cell post Immunotherapy CABozantinib)
This trial has concluded recruitment. An abstract was been submitted for ASCO GU 2024, and a poster presented.
RAMPART
Recruitment has closed internationally. Recent reports indicate that certain patients in KEYNOTE-564 had an overall survival benefit with adjuvant pembrolizumab. We are currently awaiting the publication of these results.
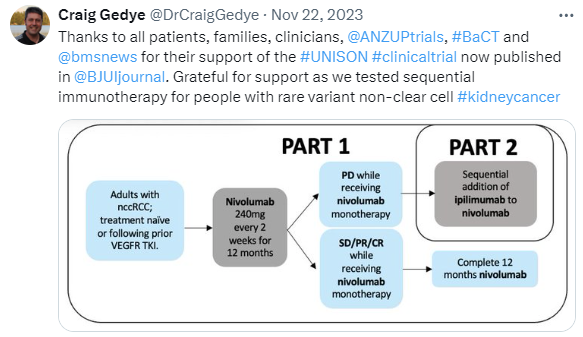
UNISoN (ANZUP 1602)
This trial has submitted a draft manuscript to BJUI, which was accepted and published: ‘A phase II trial of nivolumab followed by ipilimumab and nivolumab in advanced non-clear-cell renal cell carcinoma’.
Below the Belt Funded Projects
PREDICTing treatment responses to first line immunotherapy‐based combinations with PSMA and FDG PET in advanced Renal Cell Carcinoma
It has been submitted to HREC. some sites are considering opening both PREDICT (for first-line TKI patients) and PIRC (for second-line TKI patients).
Are ‘Dark Dimers’ Associated with Immunotherapy Benefit in Kidney Cancer?
Planning underway.
Proposals and Protocols in Development
nccRCC concepts – RADIO: Rational Dose Immunotherapy for nccRCC
We are awaiting the outcome of MRFF and CTCS NHMRC grant submissions.
Consumer-led MRFF proposal: Outcomes of Australians with Kidney Cancer (OAK)
Belinda Jago recommended initiating work on the guidelines for active surveillance of small renal cell masses prior to knowing the outcome of the MRFF funding. This will provide crucial information for the decision-making process of the study, which hinges on securing funding.
Tretinoin study
The budget has been sent to Douglas Pharmaceuticals, and we are currently awaiting their reply.
