
Chair’s Report
ANNUAL
REPORT 2024


Chair’s Report
ANNUAL
REPORT 2024

ANNUAL REPORT 2024
Chair’s Report

Ian Davis
Director and Chair of the ANZUP Board
CHAIR'S REPORT
I am delighted to present to you on behalf of the Board the ANZUP Cancer Trials Group Annual Report for 2023-2024.
ANZUP has had another eventful and highly productive year. We have continued to communicate frequently and regularly to our members, stakeholders, collaborators, and the wider community, through our newsletters and other publications. This report summarises some of our key highlights, and progress against our strategic goals and priorities.
ANZUP exists to improve outcomes for everyone affected by genitourinary cancers. We do this by designing and conducting high quality clinical trials. These are based on clinical need, as seen by those of us who work every day with our patients and our families; and are also based on the invaluable input from our Consumer Advisory Panel. Our Scientific Advisory Committee is diverse and representative of all disciplines involved in the research and care of these cancers, and provides scientific direction to the organisation. Our various subcommittees concentrate on specific disease types, or overarching themes of care and research, that give support and input into all of our work.
The Board has responsibility for running the company, including meeting all of our financial, fiduciary, and other compliance aspects. The Board comprises directors elected from the membership, and directors appointed based on their skills and expertise, to meet the skills mix required of the Board. I am very fortunate to be able to work with such a dedicated, capable, and generous group of people. Subcommittees of the Board oversee Finance and Audit, Fundraising and Partnerships, and Governance matters. The Scientific Advisory Committee and its subcommittees provides strategic scientific advice for the direction of the organisation. The Consumer Advisory Panel is pivotal and central to all that we do, providing input at all levels of our activity, including the Board, committees, and our mechanisms for generating new initiatives such as the Ideas Generation Workshops.
The Board is supported by a wonderful management team headed by our Chief Executive Officer, and the seamless and efficient nature of ANZUP activities is all thanks to them. Our administrative team organise and support all of our educational and scientific meetings, fundraising and community engagement events, and communications. Each of our trials has a governance structure underpinning it. Each trial is overseen at the highest level by a Trial Management Committee, comprising lead investigators, ANZUP staff, and coordinating centre staff. A Trial Executive Committee is a smaller group charged with managing the day to day operations and decision making for each trial. Some of our trials involve international sites, in which case we have instituted other oversight mechanisms such as International Trial Steering Committees for each relevant study; these ensure engagement of our collaborating groups and centres as well. ANZUP has established an Independent Data Safety and Monitoring Committee to ensure that independent and unconflicted expert oversight is available. We are very grateful to all who contribute to these processes.
ANZUP’s activities and directions are guided by our strategic plan, which includes the following strategic goals.
2023 saw ANZUP enter an agreement with The George Institute (TGI), and through it the University of New South Wales. This agreement brings new opportunities for all involved. One example has been the move of the ANZUP offices to a facility in Barangaroo shared with TGI, while new facilities are under construction. The link with TGI has increased our capability with new trials able to be run through them, and the appointment of a shared Fellow (Dr Carole Harris). The link with TGI also offers the potential of being able to sponsor trials in other countries where TGI has a presence.
We continue our highly productive relationships with other groups also. Many of our ongoing trials are run through the University of Sydney NHMRC Clinical Trials Centre, and this will continue to generate many valuable outcomes and opportunities in the future. We also have links with the Biostatistics and Clinical Trials Centre based at University of Melbourne; the Hunter Medical Research Institute; and others for specific projects. We have continued a very successful relationship with the Walter and Eliza Hall Institute, with whom we share a Fellow (Andrisha Inderjeeth in 2023; Anthony Uccellini in 2024). The Fellow supports not only our trials through BaCT, but also a range of other ANZUP activities such as the Ideas Generation Workshops, protocol development, manuscript writing, conference presentations, and others.
You will find our detailed financial information and audit outcomes within this report. ANZUP, as a registered charity and company limited by guarantee, is required through its Board to comply with relevant legislation and to ensure its financial sustainability. Our current main revenue streams comprise the following:
- Infrastructure support through the Cancer Australia Support for Cancer Clinical Trials program. This provides valuable support for many of our functions, and access to resources provided through Cancer Australia.
- Annual Scientific Meeting. This annual event is the key genitourinary scientific meeting in the southern hemisphere, and attracts over 400 delegates over several days of the main conference program and various affiliated meetings. Revenue arises from conference registration and sponsorship. Our 2023 theme of “Bouncing back” reflected not only our emergence from COVID isolation and lockdowns and a chance to get back together in person, but also recognised the strength and resilience of our patients, their families, and those who care for them. Our 2024 theme of “Making Waves” is not only a nod to our venue at the Gold Coast, but also relates to the effects our contributions have at a local and global level; that the experiences of our patients, their families, and those who care for them, can very much be up and down; that we can be disruptive and break complacency in the most positive sense; and also that waves can be a lot of fun.
- Corporate supporters. We are very grateful to various industry partners who generously support our activities through untied contributions to our corporate supporter program, as well as specific sponsorship of various events, meetings, and scholarships or awards. ANZUP does not take this support for granted and aims always to provide maximum value in what is a very competitive landscape for corporate sponsorship. It is very gratifying to hear from our corporate partners that their relationships with ANZUP are very strong, highly valued, and highly valuable to them.
- Our main events in recent years have been the Sydney and Melbourne Pedalthons and our #YourWay events. We also launched the ANZUP Rude Food Cookbook, with contributions from ANZUP members as well as celebrity chefs and others. All these activities contribute funds to our Below the Belt Research Fund, which is also budgeted annually by the Board regardless of the fundraising outcomes. We are very grateful to all who contribute so generously through donations or participation in these and similar events.
- This has been somewhat limited for ANZUP to date as a revenue stream, although we acknowledge extremely valuable and productive support provided through the Noel Castan Fellowships, and the Synchrony Fellowships. Individual supporters or ANZUP members also contribute financially in many ways, sometimes with donations directed to specific activities that we aim to honour wherever we can. We will shortly be launching a program aimed at tapping into people’s willingness to leave a bequest to ANZUP in their will. Impossible to value, but extraordinarily important, are the donations of time and expertise by our members and volunteers: thank you to all who contribute so generously and selflessly. Our team continues to evaluate other novel approaches to enhancing our revenue stream, and we continue to look for ways to enhance our profile in philanthropic circles.
Our financial report indicates that ANZUP’s finances are in a very healthy state. We are well placed to meet our obligations as and when they fall due, with reserves that will allow us to survive unexpected challenges, with the COVID-19 pandemic as a recent example. Resources not required for immediate use have been invested in an ethical investment portfolio managed by Perpetual, which has been delivering very good returns. The Board has placed restrictions on what assets can be included, including no direct investment in tobacco, fast food industries, or military or arms industries. The outcome of our independent audit was once again very positive.
The ANZUP administrative team does extraordinary work behind the scenes to ensure the smooth running of all that we do. This year has been particularly notable because we saw the retirement of our longstanding CEO, Marg McJannett. Marg is a nurse with extensive clinical research experience, who came to us in 2010 when ANZUP looked very different to what it is today. She oversaw extraordinary growth and achievements by our organisation, bringing together an amazing community while building a dedicated and highly capable team around her. Marg instituted a range of systems designed to ensure ANZUP’s viability and relevance. She forged critical relationships with multiple stakeholders, enabling work to be done that might otherwise not have been possible. She has challenged us all along not only to be exceptional, but to continue to strive for even greater excellence. And she has been a much-loved friend to many of us along the way. Thank you, Marg, for all that you have done and continue to do for ANZUP even in your retirement. All the very best to you and Bill in this next phase of your life, and don’t for a moment think we will ever forget you.
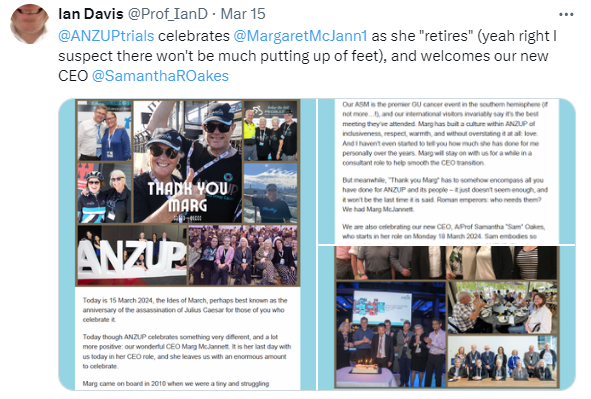
Transitions like the retirement of a CEO can be very difficult for an organisation. The Board undertook an extensive search for a replacement CEO and was delighted to appoint A/Prof Samantha (Sam) Oakes into the role. Sam brings with her extensive expertise in research and the academic and not-for-profit sectors, and her most recent position was Director, Research Investment at the National Breast Cancer Foundation. Sam is a proven experienced and capable leader, and it was immediately obvious she would be a great cultural fit to our organisation as well. Sam commenced her role with ANZUP in March 2023 and even in that short time it is clear that it is a joy to work with her. Welcome Sam!
Space does not permit me to acknowledge everybody personally, and my flawed memory will mean I am bound to miss someone if I try. I must however acknowledge Lesley Tinkler and Jo Stubbs specifically. Lesley and Jo have been tireless volunteers for us at numerous ANZUP events, particularly the Pedalthons, where their cheerfulness and boundless efficiency were infectious and energising. Lesley and Jo have stepped back from their volunteer roles, but the contributions they have made to ANZUP have been extraordinary. I am deeply grateful to them both and will miss them.

There is a lot going on. There is a lot that still needs to be done. The needs of the people we serve are still not met, although ANZUP has contributed substantially already in making a positive difference. We are continually looking to the future and need to be open to new people, new ideas, and new approaches to how we do what we do. I encourage any of you who are interested to look into what ANZUP does and what it offers, and to think about what you also might be able to contribute to this fantastic organisation. The company is in a strong position and well poised to take our next steps into the future. You will be helping us take those steps. It is a joy for me to work with all of you, and an enormous privilege that I do not take lightly. Thanks to all of you for your care for others, your generous contributions of time and expertise, and for all of your support. I am very grateful, and so are our patients, their families, and all who will come after.
I commend to you this 2023-2024 Annual Report of ANZUP Cancer Trials Group.
Ian Davis
Director and Chair of the ANZUP Board
