
Prostate Cancer Subcommittee
ANNUAL
REPORT 2024


Prostate Cancer Subcommittee
ANNUAL
REPORT 2024

ANNUAL REPORT 2024
Prostate Cancer Subcommittee
Prostate Cancer Subcommittee

Lisa Horvath
CHAIR

Jarad Martin
DEPUTY CHAIR
The Prostate Cancer Subcommittee has had an exceptionally active and productive year. Our commitment and hard work have produced notable outcomes, elaborated upon comprehensively in the subsequent report.
Updates on Trials (completed recruitment)
ENZAMET
The Health Economics sub‐study is being undertaken by the CTC and is nearing completion. PET‐MET has received HREC approval, and scans are being collected (100 expected).
Manuscripts will be developed on PSMA response and clinical progression without PSMA progression. Translational research work is underway, while long-term follow-up work is being considered.
Additionally, a poster on age-related efficacy was presented at ESMO in October 2023 in Madrid, Spain. It revealed that age did not affect the efficacy of enzalutamide.
ENZARAD
Follow-up of this study continues. Data for event-free survival analysis is expected by the end of 2024.
TheraP
Updated results from TheraP were published in Lancet Oncology – ‘Overall survival with [¹⁷⁷Lu]Lu-PSMA-617 versus cabazitaxel in metastatic castration-resistant prostate cancer (TheraP): secondary outcomes of a randomised, open-label, phase 2 trial’.
ENZA-p
Louise Emmett presented the interim data analysis findings at ESMO in 2023, which yielded positive results. The trial has subsequently been published in Lancet Oncology – ‘[¹⁷⁷Lu]Lu-PSMA-617 plus enzalutamide in patients with metastatic castration-resistant prostate cancer (ENZA-p): an open-label, multicentre, randomised, phase 2 trial’.
DASL-HiCAP
The trial is closed to recruitment in July 2023 and enrolled 1,106 people from Australia, New Zealand, Canada, US, Ireland, and the UK. Follow-up is ongoing.
EVOLUTION (Lu-PSMA vs Lu-PSMA+IO)
The EVOLUTION trial is closed to recruitment and enrolled 93 people from across Australia. Planning is underway for translational research work with biomarker samples.
#UpFrontPSMA
The recruitment is completed with 130 patients enrolled across Australia, with the study now in the follow-up phase. The study has been published in BJUI – ‘UpFrontPSMA: a randomized phase 2 study of sequential 177Lu-PSMA-617 and docetaxel vs docetaxel in metastatic hormone-naïve prostate cancer (clinical trial protocol)‘.
Updates on Recruiting Trials
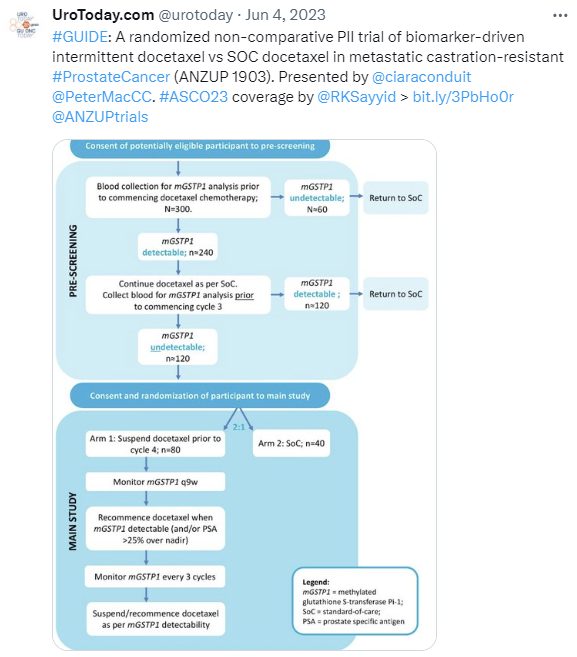
GUIDE
Recruitment is progressing slowly, with only 6 patients randomised as of 31 March 2024. We will explore an adjusted recruitment target.
PRIMARY2
Recruitment is progressing well.
ANZadapt
23 patients have been recruited locally, with all 10 sites open. Additionally, 14 patients were recruited by Dutch collaborators. We are currently in discussions with the Dutch group to amend the entry criteria for PSA to 2.0 to boost local recruitment.
NINJA
The recruitment is progressing well with 319/472 patients accrued, aiming to complete accrual in late 2025.
The decision-making process for men with rising PSA post radical prostatectomy after 10 years who have metastasis identified after PSMA PET-CT scan
18 patients have been recruited and 16 interviews have been completed. Recruitment continues to ensure a diversity of views is obtained.
Studies in Start-up
GenI-AIRSPACE: Genomically Informed Active Surveillance in Favourable Intermediate Risk Prostate Cancer
HREC approval has been received, and we are finalising service agreements. The feasibility survey will be re-circulated once service agreements are finalised.
WOMBAT: High-dose testosterone and darolutamide re-sensitisation following asymptomatic PSMA failure on darolutamide (for CRPC)
We are currently finalising service agreements, with the PICF already submitted to HREC. A response has been received, and approval is expected soon. Additionally, database development is in progress.
Substudies
ENZAMET Translational Research
SNIP, plasma cytokine and lipidomics analysis of samples have been completed, and data are being reviewed. Samples for germline analysis have been sent to the University of Melbourne, and samples for other analyses will be sent to Veracyte.
ENZAMET PET MET
Louise Emmett will analyse scans once we receive scans from Frankston, Hobart, and PMCC. Negotiations are underway with Emory University for radiomics analysis.
ENZARAD Translational Research
Edmond Kwan will oversee the scope of work. Members are encouraged to submit proposals for translational projects.
ENZA-p
Imaging analysis is underway. Samples have been sent to repository for ctDNA and lipidomics analyses.
EVOLUTION Translational Research
A translational research plan has been developed and we are investigating potential funding sources.
Concepts in Development
DARO-LIPID
Tahlia Scheinberg will be a co-investigator. We have secured a grant from the Ramsay Hospital Research Foundation and are currently seeking additional funding. The protocol is currently under development, and we plan to send out feasibility surveys to sites.
PAIN FREE TP
The NHMRC/Cancer Australia grant application was unsuccessful. We are considering resubmitting for a philanthropic grant, which could include applications to the Ramsay Hospital Research Foundation and PCFA.
PEACE-7
The protocol is nearing finalisation, including input from ANZUP. A final decision will depend on securing sufficient funding.
KUNG-FU: Fezolinetant study
Proposed study aims to reduce ADT-related hot flashes.
