
Goal 2
ANNUAL
REPORT 2024


Goal 2
ANNUAL
REPORT 2024

ANNUAL REPORT 2024
Goal 2
Goal 2: Maintain a portfolio of trials relevant to and accessible by all people with urogenital cancers in Australia and New Zealand
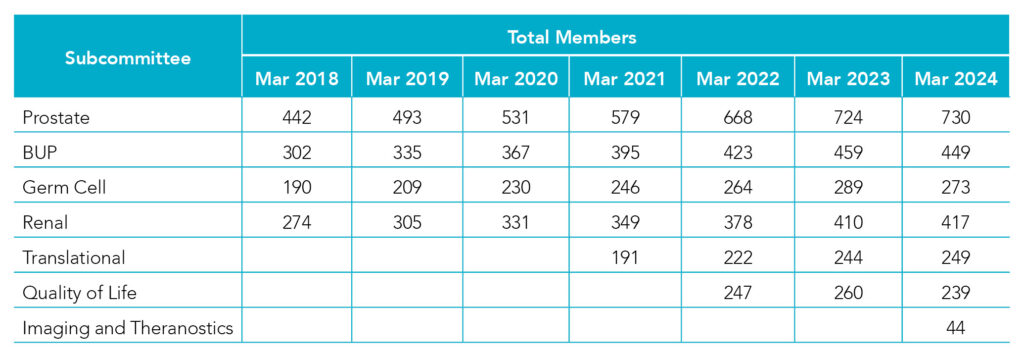
ANZUP is committed to raising awareness, fostering participation, and ensuring access to ANZUP trials through various channels. Our subcommittee membership continues to grow across all cancer types and areas of interest.
We actively encourage attendance at subcommittee meetings, extending a warm welcome to both regular attendees and newcomers, including colleagues and trainees.
To underscore the advantages of ANZUP membership, we actively engage in external meetings such as ANZUNS, the USANZ New Zealand Section Meeting, COSA, and the Asia Pacific Advanced Prostate Cancer Symposium.


Throughout the year, ANZUP saw the participation of 16 rural/regional sites in trials, with 23 patients recruited. Rural members and their patients are often invited to contribute content for our clinical newsletter, UPdate, and consumer magazine, ‘A Little Below the Belt’, distributed to over 400 cancer centres, including 28 rural/regional sites.
The Consumer Advisory Panel (CAP) is involved in all ANZUP research initiatives, spanning the Scientific Advisory Committee (SAC), subcommittee meetings, Idea Generation Workshops (IGWs), and the Annual Scientific Meeting (ASM). We ensure CAP membership reflects a diverse array of disease types, guaranteeing meaningful patient and caregiver perspectives in our decision-making processes.
Community engagement is reinforced through the Community Engagement Forum (CEF), where consumers are invited to participate. Held at the #ANZUP23 ASM, the CEF offers a platform for discussing the importance of clinical trials and raising awareness about ANZUP’s mission, as well as the impact of below the belt cancers on individuals and families. These forums are also recorded and shared with a wider audience.

ANZUP utilises various social media platforms — X (Twitter), Facebook, Instagram, LinkedIn, and YouTube—to educate and connect with the broader community regarding our activities, events, and clinical trials.
