
Chief Executive Officer’s Report
ANNUAL
REPORT 2024


Chief Executive Officer’s Report
ANNUAL
REPORT 2024

ANNUAL REPORT 2024
Chief Executive Officer’s Report

Samantha Oakes
Chief Executive Officer, ANZUP
CHIEF EXECUTIVE OFFICER'S REPORT
ANZUP Cancer Trials Group Ltd is committed to our mission of improving the lives of people affected by bladder, kidney, testicular, penile, and prostate cancers, through practice-changing multidisciplinary collaborative clinical trials. These trials generate evidence for more effective treatments that can make a real difference in the lives of those impacted by these cancers. We continue to take a patient-focused approach to our trials and remain committed to improving the outcomes of people diagnosed with Below the Belt cancers but maintaining and or improving quality of life and survivorship.
Having the privilege of being appointed as the new Chief Executive Officer (CEO) of ANZUP, I reflect on the last 12 months masterfully led by our retiring CEO, Ms Margaret McJannett.
Our work has continued to garner global recognition for its significance and transformative impact. With 33 clinical trials either complete, in development, actively recruiting or in follow-up, over 8,000 participants have been enrolled in ANZUP clinical trials in over 750 distinct sites worldwide, our reach is both extensive and impactful.
Over the past year, ANZUP has continued to achieve significant milestones, thanks to the exceptional dedication of our members.
Our clinical trials research highlights include:
ANZUP is constantly expanding its portfolio of high-quality and cutting-edge clinical trials, with a focus on the cancers we prioritise. Additionally, we are thrilled to unveil the development of several new trials, which will advance to opening throughout 2024/2025.
In line with our Strategic Plan, ANZUP actively fosters multidisciplinary collaboration, including, at all stages, input from our Consumer Advisory Panel, to propose new trial ideas. Our Ideas Generation Workshops (IGWs) provide a vital platform to examine treatment evidence, identify gaps, and ensure patient centred approaches to critical clinical questions. These workshops benefit experienced researchers and offer valuable opportunities for future leaders. ANZUP encourages participation from fellows, trainees, and junior researchers. Currently, IGWs are ongoing across all subcommittees.
We continue to deliver on the annual activities and expected outcomes of our Support for Cancer Clinical Trials 2022 – 2024 infrastructure funding by identifying and developing cancer clinical trial protocols that address the most important clinical questions in the healthcare system and strive to reduce disparities in cancer outcomes, including among Aboriginal and Torres Strait Islander and culturally and linguistically diverse communities.
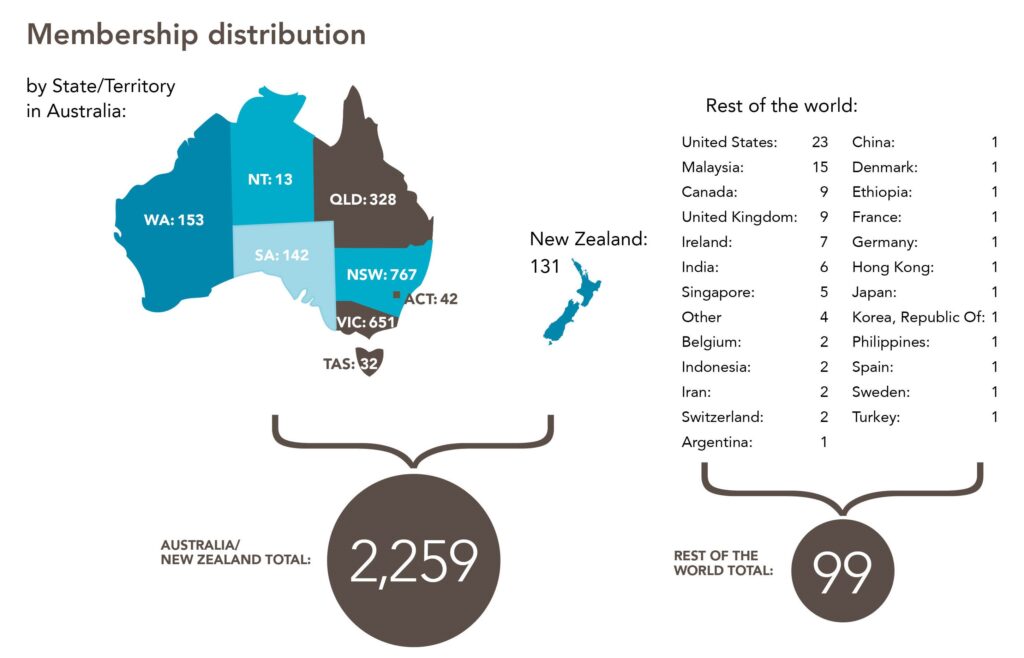
ANZUP’s membership has steadily expanded, surpassing 2,300 individuals across diverse disciplines and professional groups as of 31 March 2024.
Our #ANZUP23 Annual Scientific Meeting took place from 9 – 11 July 2023 at the Melbourne Convention Centre, marking the highest attendance yet with 446 delegates in attendance. With the theme of ‘Bouncing Back’, we talked about how people and systems have adjusted and changed in response to the pressures of the pandemic, and in relation to the impacts of Below the Belt cancers. We had more than 80 speakers, panelists, session chairs, and poster presenters, including the outstanding international faculty of Andrea Apolo, Laurien Buffart, Ananya Choudhury, Darren Feldman, Rebecca Martin, Sima Porten, Bertrand Tombal, and Alex Wyatt.


Additionally, we celebrated the return of our Sydney Pedalthon on 31 October 2023, where over 120 registered riders joined, collectively raising over $75,000. None of our achievements would be possible without the invaluable support of our community members. Thank you for your unwavering dedication.
Strategic & Business Planning
In 2023, ANZUP continued to deliver on the five objectives under our Strategic Plan:
- Conduct high-quality, multidisciplinary, practice-changing clinical trials in urogenital cancers
- Maintain a portfolio of trials relevant to and accessible by all people with urogenital cancers in Australia and New Zealand
- Strengthen ANZUP’s capacity for practice-changing clinical trials
- Forward plan to maintain a vibrant and active urogenital cancer trials community
- Provide leadership in collaborative cancer clinical trials
Supported by our dedicated membership, key stakeholders, and the wider ANZUP community, we stand firm in our commitment to pursue these objectives, driven by our overarching mission to improve the lives of people affected by bladder, kidney, testicular, penile and prostate cancers through practice-changing multidisciplinary collaborative clinical trials.
Throughout the year, we maintained a consistent schedule of meetings with various committees and subcommittees, including the Finance and Audit Committee, Fundraising and Promotion Subcommittee, Operations Executive, Scientific Advisory Committee (SAC), Subcommittees (by disease type), Consumer Advisory Panel (CAP), and Trial Management Committees (TMC). These meetings help to ensure that all our ANZUP activities align harmoniously with our Mission and Strategic Plan, enabling us to make informed decisions and drive progress towards our goals.
In 2024, ANZUP will embark on developing our next Strategic Plan to continue to deliver on our mission through a broad consultation with our stakeholders including the Board, our staff, committees and subcommittees, membership, partners and collaborators and consumer advisory panel.
Finances
ANZUP expresses gratitude for the ongoing infrastructure funding provided by the Australian Government through Cancer Australia. While we deeply appreciate this support, it’s crucial to note that each clinical trial requires independent funding. Therefore, ANZUP is dedicated to achieving greater financial autonomy and sustainability by exploring diverse fundraising avenues.
We remain fully committed to seeking sustainable and innovative funding sources to initiate trials and support our members in developing concepts and trials in Below the Belt cancers. We extend our sincere thanks to our corporate supporters, sponsors, and donors for their continued support and generosity. With their invaluable assistance, ANZUP will be able to discover improved treatments and outcomes for our patients.
ANZUP’s strong financial position allows us to maintain our investment in research. We will continue to exercise fiscal prudence in managing our budget and investment strategy to ensure the longevity of our critical research endeavours. A strong balance sheet empowers ANZUP to seize significant opportunities as they emerge.
For a more comprehensive overview of ANZUP’s finances, please refer to our financial report available here.
Data and quality
ANZUP works closely with our coordinating centres, including the NHMRC Clinical Trials Centre (CTC), the George Institute for Global Health (TGI) at UNSW Sydney, the Centre for Biostatistics and Clinical Trials (BaCT), the Walter and Eliza Hall Institute of Medical Research (WEHI) and the Hunter Medical Research Institute (HMRI). Together, we establish robust processes for trial development and operations. These organisations implement quality management systems, standard operating procedures (SOPs) and templates to ensure accuracy and consistency throughout the trial process.
Before launch, data systems undergo meticulous planning, programming, and thorough checks to ensure reliability. Additionally, monitoring, and formal audit processes are in place to support these efforts. ANZUP also conducts annual training for significant site staff members to ensure the maintenance of data quality. All procedures and data systems adhere to national and international guidelines for clinical trial conduct.
To further ensure the safety and well-being of patients participating in ANZUP trials, we have established an Independent Data and Safety Monitoring Committee (IDSMC). The IDSMC evaluates the plausible benefits and risks associated with patient participation in ANZUP trials and assesses whether these trials should continue as per their original design. The IDSMC regularly reviews trial data and communicates findings to the ANZUP SAC Chair and the relevant Trial Management Committee (TMC).
Staffing
We want to express our gratitude to our dedicated and diligent management team, who tirelessly support our members, key stakeholders, and the broader community. Continuously evaluating our current capacity, we’ve ensured that our team possesses the necessary expertise and resources to excel in critical areas such as clinical trials management, database coordination and support, internal and external communications, event production and promotion, fundraising and corporate partnerships, and administrative support.
We would like to express our sincere gratitude to our long-serving and dedicated volunteers, Lesley Tinkler and Jo Stubbs. Their invaluable contributions over the past 12+ years have bolstered our operations, enabling us to create positive impacts within the community.
Our sincere appreciation extends to our hardworking ANZUP Fellows, Dr Carole Harris, Dr Andrisha Inderjeeth and Dr Anthony Uccellini, and our colleagues at NHMRC CTC at the University of Sydney, TGI at UNSW Sydney, BaCT, WEHI, and HMRI. Thank you for your support and invaluable contributions.
Education and mentoring
ANZUP remains committed to enhancing education and mentoring opportunities for our members. Through a diverse array of educational events, we aim to enrich their knowledge and continuously cultivate innovative clinical trial ideas. Recognising the significance of nurturing the next generation of scientific and clinical leaders, we prepare them for the future landscape of clinical trials.
Throughout 2023 and 2024, we held our Ideas Generation Workshops both in person and via Zoom, drawing 115 participants and showcasing 27 pioneering concepts to our multidisciplinary community. These workshops are where many of the “seeds” for ANZUP clinical trials are sown, and they are important to grow and foster a pipeline of innovative ideas to be considered for support from ANZUP to progress.
In November 2023, we had our ANZUP GU Cancer Rapid Fire Program in Melbourne. This multidisciplinary educational program has been developed to facilitate further understanding of contemporary oncology management through advances in clinical trials. It covers landmark clinical trials in GU cancer while providing mentorship from experts in the field. The program has been designed to offer trainees and junior consultants’ insight into the evolution of GU cancer management and the evidence behind contemporary clinical practice. Special thanks go to Co-convenors Andrisha Inderjeeth and Ciara Conduit for their invaluable contributions.


Collaborations
ANZUP is proud of our strong collaborations with national and international research groups, showcasing our steadfast dedication to improving patient treatment and outcomes.
We are delighted to work alongside dedicated investigators and trial staff, as well as our colleagues at CTC at the University of Sydney, TGI at UNSW Sydney, BaCT, WEHI and HMRI.
In August 2023 we announced a new collaboration between ANZUP and TGI. TGI is affiliated with the University of New South Wales (UNSW Sydney) and has extensive experience in the conduct of national and international clinical trials investigating treatments for chronic and critical conditions. Together with ANZUP’s world-leading multidisciplinary members, including our community representatives, we continue to build our capacity and capability to improve treatment and outcomes for people affected by genitourinary cancers. To further develop and strengthen this partnership and with ANZUP’s expanding team and the limited office space, we moved with TGI to their temporary new location at Barangaroo in December 2023 as we wait for new offices to be built at UNSW scheduled to be around late 2025.
We extend our deepest appreciation to our corporate supporters, sponsors, and donors for their continuous generosity and support. Their contributions play a pivotal role in enabling ANZUP to advance its mission of enhancing treatments and outcomes for those fighting Below the Belt cancers.
We sincerely thank the thousands of patients who participate in our ANZUP trials. Your invaluable contributions enable us to fulfil our mission to improve the lives of people affected by bladder, kidney, testicular, penile and prostate cancers through practice-changing multidisciplinary collaborative clinical trials.
Finally, we acknowledge the incredible contributions and legacy left by our outgoing CEO, Ms Margaret McJannett, who transitions to semi-retirement and to sunnier days in Queensland. Marg committed over thirteen years as the CEO of ANZUP and together with the Board of Directors, led the transformation of ANZUP to one of the leading collaborative clinical trials groups in Australia. Under Marg’s leadership, ANZUP has made significant progress in the fight against Below the Belt cancers, advancing research, and providing vital support to patients and their families. While I will never replace Marg, I am committed to building on her legacy and know that with the support of the ANZUP leadership team from the Board, SAC, Subcommittees, CAP and membership, we can continue to improve the lives of those affected by Below the Belt Cancers. I am incredibly privileged to have been given the honour to lead our wonderful organisation into the future. I am dedicated to delivering on our Strategy, developing our new growth Strategy in 2024, and know that, together, we will continue to thrive and make a significant impact in the years to come. Thank you all for your unwavering commitment to our mission.
I commend ANZUP’s 2023/2024 Annual Report to you.
A/Prof Samantha R. Oakes
Chief Executive Officer, ANZUP
