ANZUP at ESMO 2025 – ENZA-p
ENZA-p
Background
Prostate cancer remains the most commonly diagnosed cancer in Australia and New Zealand with over 28,000 cases expected to be recorded in 2025¹,². Thanks to world class research, prostate cancer can be treated effectively for the majority of the people affected. Nevertheless, over 4,000 people every year will die due to an advanced or hard-to-treat form of prostate cancer and many more people will have life-long treatment related side-effects including morbidity associated with long-term hormonal suppression, sexual dysfunction and mental health issues³.
Around 96%¹ of people diagnosed with prostate cancer will survive to their five-year milestone but for those with advanced (metastatic) and hard-to-treat cancer, this number is much lower at around 40%³. Better treatments for advanced and hard-to-treat prostate cancers are desperately needed. While hormone treatments like enzalutamide, which block the effects of testosterone, can slow cancer growth in people with advanced prostate cancer, unfortunately, some people will develop resistance to the treatment and around 1 in 4 people will never respond.
About the trial
The world first ENZA-p study, led by Professor Louise Emmett, tested whether a powerful new combination of LuPSMA combined with enzalutamide could improve outcomes in people diagnosed with high-risk advanced (metastatic) castration-resistant prostate cancer.
The trial also tested an innovative world-first treatment approach called ‘adaptive dosing,’ which uses imaging and blood results to identify patients who are responding to the treatment and determine those patients who were most likely to benefit from continued treatment, tailoring the treatment approach for each patient.
Poster presentation at ESMO 2025
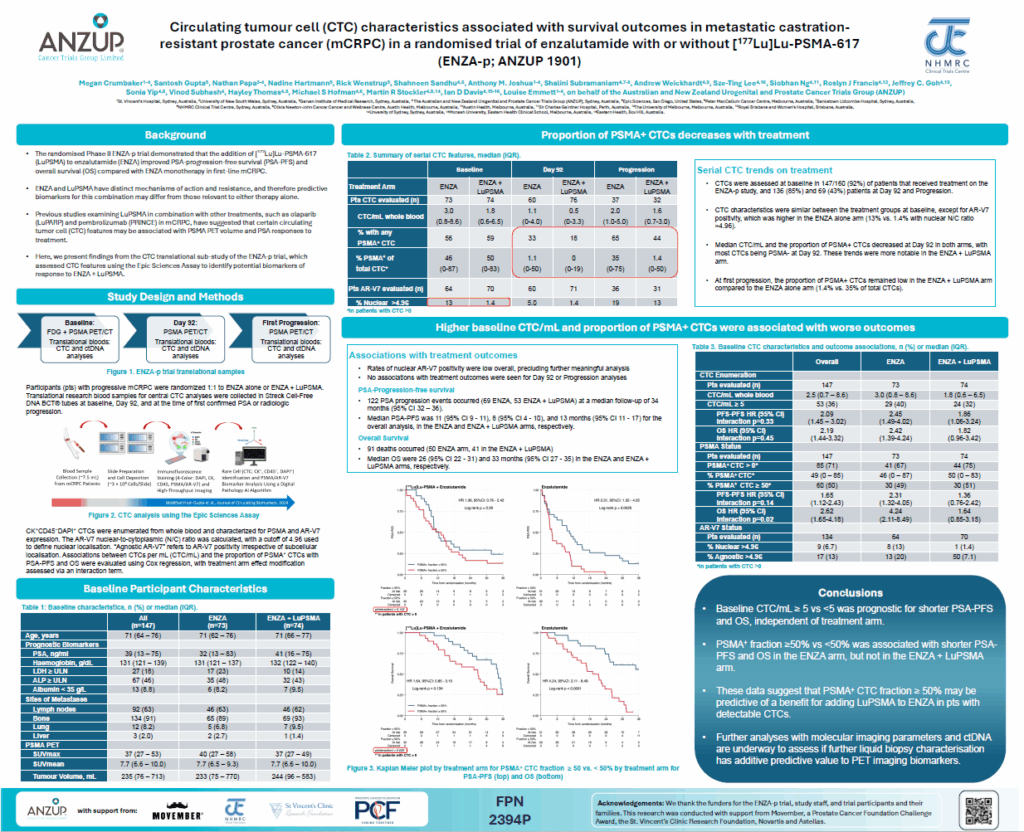
Circulating tumour cell (CTC) characteristics associated with survival outcomes in metastatic castration-resistant prostate cancer (mCRPC) in a randomised trial of enzalutamide with or without [177Lu]Lu-PSMA-617 (ENZA-p; ANZUP 1901) – Presented by Megan Crumbaker
This translational sub-study of ENZA-p evaluated CTC features as biomarkers of response to enzalutamide (ENZA) ± LuPSMA.
CTCs were measured at baseline in 147 out of 160 patients who received their treatment in ANZUP’s ENZA-p study, which has provided convincing evidence of the benefit of adding LuPSMA to enzalutamide in mCRPC. Having 5 or more circulating tumour cells (CTCs) per millilitre of blood at the start of treatment was linked to shorter progression-free and survival, independently of whether patients received enzalutamide alone or in combination with LuPSMA.
Among patients treated with enzalutamide alone, those who had 50% or more of their baseline CTCs positive for PSMA expression were more likely to have shorter overall survival but this effect was not seen in the enzalutamide plus LuPSMA arm, suggesting that the effect of high PSMA expressing CTCs on overall survival was modified by the administration of LuPSMA.
These results suggest that high levels of PSMA expressing CTCs (more than 5 CTCs/ml and with greater than 50% expressing PSMA) may be predictive of benefit to LuPSMA added to enzalutamide.
References:
- Cancer Data in Australia, Australian Institute of Health and Welfare (AIHW) 2025
- New Zealand Cancer Registry (NZCR), Health New Zealand-Te Whatu Ora
- National Cancer Control Indicators; relative survival for prostate cancer by stage, 2011
