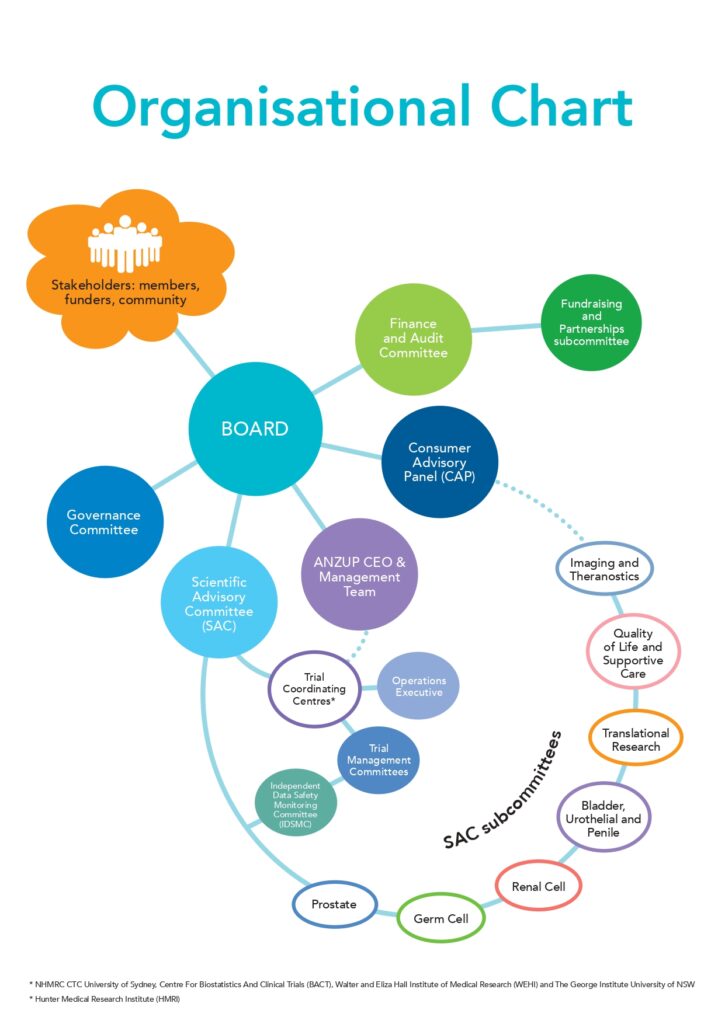
Our Governance
The Board comprises the Directors of the Company and is responsible for financial management, corporate governance,
reporting and compliance. The Board consists of five elected Directors and four Appointed Directors.
Finance and Audit Committee
A committee of the Board. Its main objectives are to assist the Board in the discharge of its responsibility to exercise due care, diligence and skill; and to provide a formal forum for financial management, compliance and control.
Fundraising and Partnerships Subcommittee
A subcommittee of the Finance and Audit Committee. Its main objectives are to identify and pursue opportunities
for additional revenue through fundraising and production of relevant marketing materials for ANZUP.
Management Team
The Management team comprises the Chief Executive Officer, Executive Assistant and Office Manager, Marketing Manager, Communications Manager, Marketing and Communications Specialist, Communications and Event Co-ordinator, Business and Philanthropy Manager, Database Specialist, Clinical Trials Operations Manager, Translational Research Operations Manager, Senior Clinical Trials Project Manager, Clinical Trials Project Managers and Clinical Trials Assistant. The office is supported by volunteers. The company’s registered office is in Sydney.
The SAC consists of a core of members representing the major disciplines relevant to ANZUP, nominated and appointed upon the recommendation of those groups. In addition, Chairs of the SAC subcommittees are members of the SAC by virtue of their appointment as Chair. The SAC meets by teleconference quarterly with one annual face-to-face meeting during the ASM.
SAC subcommittees
The SAC is advised by disease specific subcommittees (Prostate, Renal, Germ Cell and Bladder/Urothelial/Penile) and non-disease-specific subcommittees (Quality of Life & Supportive Care, Translational Research and Imaging & Theranostics). The disease specific subcommittees are responsible for oversight of trials within their portfolios, as well as development of new trial concepts. These subcommittees meet by teleconference quarterly and intend to meet face-to-face at least once per year. The non-disease-specific subcommittees are involved as required in trial development and management in order to ensure maximum value is added to every trial.
The ANZUP CAP reports to the Board. It comprises consumer/community representatives who contribute at all levels of governance, from the Board and SAC and its subcommittees through to specific trials and research projects. The CAP also provides a conduit for communication from ANZUP back to the community in order to promote clinical trial research. The CAP meets by teleconference quarterly and intends to meet face-to-face at least once per year where resources permit.
Operations Executive Committee
This committee consists of representatives from ANZUP and each of its coordinating centres – the NHMRC Clinical Trials Centre at The University of Sydney and the Centre for Biostatistics and Clinical Trials. The committee is responsible for oversight of trials and group operations.
Independent Data Safety Monitoring Committee (IDSMC)
The broad aim of the IDSMC is to evaluate the plausible benefits and risks associated with patient participation in ANZUP trials. The IDSMC comprises at least three members who are experienced in clinical research and are not conflicted with ANZUP. The committee oversees a number of ANZUP studies and co-opts others onto it when additional advice is required. The IDSMC advises the relevant Trial Management Committee (TMC) Chair(s), Group Chair and Scientific Advisory Committee Chair.
Trial Management Committees
Each trial has a TMC that meets approximately quarterly by teleconference to ensure oversight of the trial.